AI in MedTech #2: Insights from 882 device approvals through March 2024
In a recent update, FDA published a list of 882 AI/ML enabled medical devices authorized since 1995 through March 2024. Here are 5 surprising observations about AI applications in medical devices.
The FDA recently updated a list of artificial intelligence/machine learning (AI/ML) enabled medical devices marketed in the United States1. The updated list now provides key details for 882 medical devices that have been approved or cleared by the FDA as of March 2024.
This article offers 5 surprising observations based on the analysis of data in the updated list.
1. FDA approved the first AI/ML device in 1995, but the pace began accelerating only after 2016.
There is a lot of buzz about AI these days, but did you know that the US FDA approved the first AI/ML enabled medical device in 1995? It was an AI-enabled cervical smear rescreening device for rapid screening with better sensitivity2. Since then, there has been rapid innovation in this space.
As shown in Figure 1 below, the pace of AI/ML device approvals3 increased rapidly starting in 2016. Until that point, FDA had authorized only 33 AI/ML enabled devices for marketing in the US. In the 7 years following 2016, AI/ML device approvals increased at compounded average annual rate (CAGR) of 49%, reaching a total of 837 by 2023.

As of the latest update through 29 March 2024, a total of 882 AI/ML enabled devices have been approved, cleared or reclassified as Class II devices. At the current growth rate, this number is expected to grow over 1000 total AI/ML devices approved by the end of 2024.
2. Radiology continues to dominate the category, but Cardiovascular applications are growing fast.
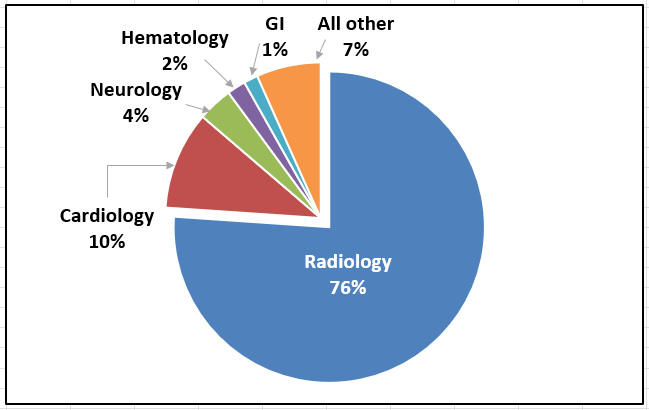
As shown in Figure 2, 76% of devices AI/ML approved (671/882) so far have been in the Radiology medical specialty. The other top specialties are: Cardiovascular, Neurology, Hematology and Gastroenterology-Urology (GI). These top 5 medical specialties account of 93% of the total AI/ML devices approved.
There has been a noticeable growth in Cardiovascular applications since 2015 as shown in Figure 3 below. The first AI/ML device for a cardiovascular application was cleared in 2015. Since then, the category has grown at a CAGR of 75% to reach a total of 90 AI/ML devices cleared by March 2024.

3. AI/ML applications span across a broad range of medical devices.
Just as diverse medical devices are in their intended use applications, so are AI/ML enabled devices in their applications across different medical specialties.
As shown in Figure 4, top 3 AI/ML device codes in Radiology, Cardiovascular and Neurology specialties span a broad range of medical applications, ranging from radiological image processing to blood flow visualization to monitoring brain activity. In nearly all cases, these devices are used to assist in diagnosing a medically significant condition or planning an appropriate medical treatment for a specific patient.
4. Novel applications without a predicate device are growing fast.
A vast majority of the AI/ML devices (97%; 856/882) have been cleared through the 510k premarket notification pathway. Only 4 required a PMA and 22 were reclassified as Class II through the De Novo pathway.
As shown in Figure 5, Cardiovascular, Radiology and Neurology medical specialties collectively accounted for 64% of the De Novo reclassifications (14/22) granted for AI/ML devices from 2013-2023.
A total of 7 De Novo were granted in 2021, the most in any single year, with 4 in the Cardiovascular specialty alone. Among these 4 was the breakthrough Feops HEART guide (DEN 200030), an interventional cardiovascular implant simulation software4.
In the same year, another innovative AI/ML enabled device to aid in the detection of pediatric autism spectrum disorder (ASD)5 received marketing authorization through the De Novo pathway.
Although not yet high in quantity, these novel AI/ML enabled devices highlight the future of this technology in assisting with diagnosis and treatment of challenging medical conditions.
5. Novel AI/ML devices are fueling rapid innovation in their respective application spaces.
Devices reclassified through the De Novo process are evaluated on the basis of benefit-risk. If cleared, the FDA creates a new device category with its own regulation and special controls.
As a result, a De Novo device serves as a predicate device for other substantially equivalent devices to enter the market through the less burdensome 510k pathway.
As an example, DEN170073 granted in 2018 to ContaCT, a radiological computer-assisted triage and notification software6, led to a total of 52 new Ai/ML 510k’s to other similar devices in the following 6 years.
Another example is DEN17022 granted in 2017 to QuantX7, a computer-assisted diagnostic software for lesions suspicious of cancer, led to 9 new AI/ML 510k’s for other similar devices in the following 7 years.
Similarly, in the Cardiovascular specialty, DEN200019 granted in 2021 to Oxehealth Vital Signs8, a software for optical camera-based measurements of pulse rate, breathing rate, and/or respiratory rate, led to 3 new AI/ML 510k’s for other similar devices in short span of only 2 years.
In conclusion
The future of AI/ML applications in the medical device industry is bright!
These technologies are helping physicians and patients find new ways to manage challenging medical conditions. Medical device innovation has been rapidly increasing since 2016, with a total of 882 AI/ML devices receiving FDA approval through March 2024.
But these numbers don’t tell the whole story. At a high level, Radiology specialty dominates the number of AI/ML devices approved, but novel devices in the Cardiovascular and Neurology specialty are on the rise. Once approved, some of these novel devices are also fueling rapid growth by serving as predicates for other similar devices that can get to market through a less stringent 510k regulatory pathway.
This is good news for physicians and patients.
FDA: Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices, Updated 13 May 2024.
FDA: P940029 PAPNET (R) Testing System, PMA database entry.
Note that we are using the term “approval” interchangeably with marketing authorization. FDA approval specifically applies to the PMA regulatory pathway used for high risk
Class III devices. Most of the AI/ML enabled devices have been “cleared” through the 510k pathway, or reclassified as Class II devices through the De Novo process.
See a brief review of the FEops HEARTguide.
See a brief review of the Cognoa ASD diagnosis aid.