How artificial intelligence is advancing medical device innovation
A review of 5 innovative devices recently approved by the FDA
There is a lot of buzz about artificial intelligence and machine learning (AI/ML) these days, but did you know that the US FDA approved the first AI/ML enabled medical device in 1995? Since then, there has been an exponential rise in the number of AI/ML enabled devices cleared by the FDA, mainly through the premarket notification1 process. According the most recent list published by the FDA2, a total of 521 AI/ML enabled devices have been approved or cleared since 1995.
A vast majority of AI/ML enabled devices fall under the Radiology category and broadly used for enhanced image processing to facilitate clinical decisions. The top 5 medical specialties collectively account for about 93% of the AI/ML enabled devices.
The top 10 product codes, as shown in Figure 3 below, account for a total of 342 (~66%) of the AI/ML enabled devices. Each product code and device type identifies a generic category of a medical device regulated by a specific regulation number3.
Here are 5 examples of innovative AI/ML devices recently cleared by the FDA.
Innovative AI/ML enabled device example #1 (Radiology)
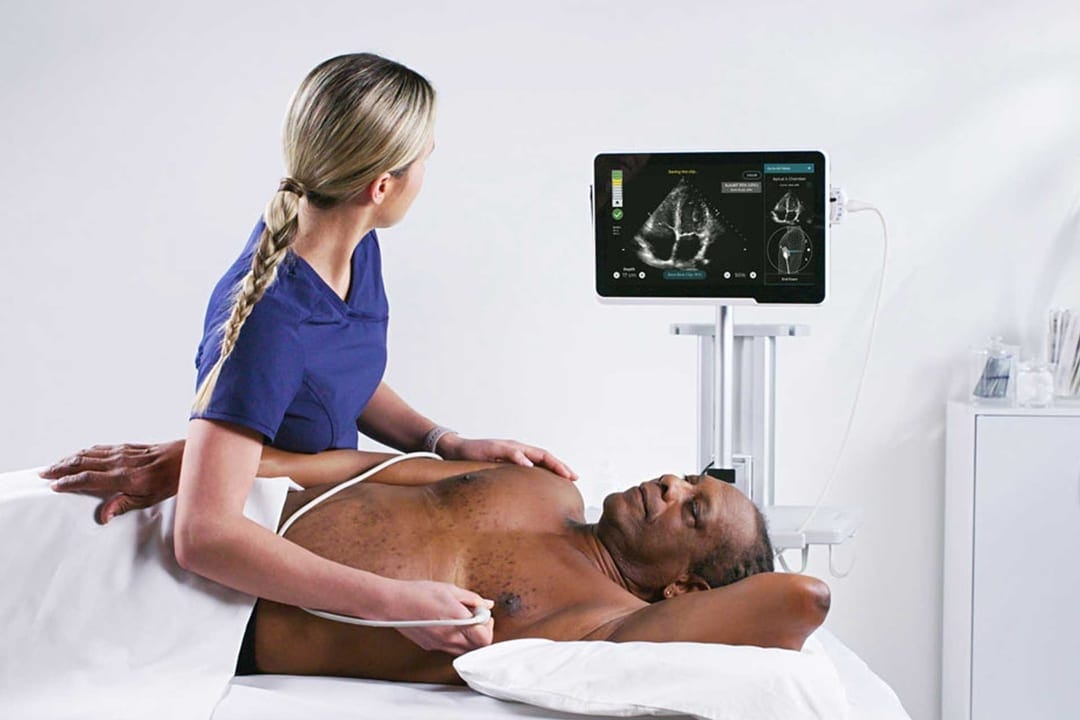
Caption Guidance: AI-guided ultrasound system software
It takes special training and experience to acquire high quality ultrasound images during an echocardiogram (ECG). Limited availability of trained staff and imaging centers limits access and early detection of serious heart conditions. Imagine the impact if the software could guide a nurse or medical staff without specialized training to move and position the transducer correctly and provide real-time feedback on image quality so they could adjust the position? A routine ECG could be done even in remote areas where specialized care may not be available. Potentially serious heart conditions could be detected early and many more patients could benefit from a timely, lower cost intervention.
This is the promise of the innovative Caption Guidance4 software, which is used as an accessory to a compatible general purpose ultrasound diagnostic system to assist minimally trained medical professionals in completing an ECG with good quality images. The FDA fast-tracked this device through the breakthrough device program reserved for more effective treatment or diagnosis of life-threating diseases or conditions5.
A pivotal study with 8 nurses6 trained on the Caption Guidance system demonstrated the clinical utility for users without specialized ECG training. A panel of five expert cardiologists evaluated the Caption Guidance assisted scans obtained by the nurses on 30 patients to assess the image quality against 10 clinical parameters. Results showed that image quality of the scans by nurses was sufficient to make clinical assessment against 4 primary criteria in more than 90% of scans. These primary clinical criteria included the assessment of left ventricular size, global left ventricular function, right ventricular size and non-trivial pericardial function. The image quality was considered to be of “diagnostic quality” in a high proportion of clips in each of the 10 standard views.
The auto-capture functionality was utilized in about 46% of the clips acquired by the nurses. About 94% of these clips were rated as diagnostic quality by the panel of cardiologists.
A human factors study with 5 user groups including physicians, nurse practitioners, physician assistants, registered nurses, medical residents and certified medical assistants showed that 100% of the critical tasks were completed without any errors. No other use-errors were found that could cause harm to the scanner or the patient.
Innovative AI/ML enabled device example #2 (Cardiovascular)
FEops HEARTguide: Interventional cardiovascular implant simulation software
Imagine if your surgeon could first study a “digital twin” of your heart before performing a life-critical cardiac intervention such as left atrial appendage occlusion (LAAO) device implantation. Think of these devices as a “plug” to prevent blood clots from entering the circulatory system in patients with atrial fibrillation (AFib7) who may develop clots in the left atrial appendage (LAA8). Blood clots in the system is a significant concern for patients with AFib because they can enter the brain resulting in a stroke or even death.
The LAAO device implantation9 is a complex, high-risk procedure. It requires careful planning to select the right-sized device for an individual patient to ensure proper placement and sealing. Anatomical differences in the heart as well as the venous system used to access the heart can lead to many surprises during the procedure and sub-optimal outcomes. A digital twin of a patient's heart generated using AI provides a powerful tool for planning the procedure and optimizing the results.
The HEARTguide is a prescription-only medical device indicated for patient-specific simulation of transcatheter LAAO device implantation during procedural planning10. This AI-driven software can be used to predict the implant frame deformation to support the evaluation of the LAAO device size and placement.
A 200-patient PREDICT-LAA11 prospective, multi-center, randomized controlled trial studied the effect on procedural efficiency and outcome. The results were impressive12:
Efficiency: 2x procedural success with single device and deployment; 25% reduction in use of radiation and contrast medium and 100% procedural success with no complications in the simulation group
Outcomes: 40% higher complete LAA closure with no leakage, 60% lower retraction of Amulet(TM) disc into the LAA and 80% less risk for device-related thrombus (DRT).
Innovative AI/ML enabled device example #3 (Neurology)
Cognoa ASD diagnosis aid for pediatric autism spectrum disorder
Autism spectrum disorder13 (ASD) is fast emerging as one of the most significant concerns in early childhood development. Many children continue to remain undiagnosed until they are much older, growing up with the pain and social stigma of this disability.
Imagine the positive impact on a child’s life of an early diagnosis and timely intervention! Did you know that some children show ASD symptoms within the first 12 months of life? In others, symptoms many not show up until later. On average, the average age of ASD diagnosis is 4 years and 3 months, which represents an average delay of nearly 3 years when initial symptoms appear.
Cognoa ASD diagnosis aid14 is an AI-powered mobile app intended for use by healthcare providers as an aid in the diagnosis of ASD for patients ages 18 months through 72 months, considered to be at risk for development delays by their parents, caregivers or pediatricians.
This app gives a simple Yes, No or Indeterminate finding to ASD based on analysis of 2 observational videos of the child and short questionnaires completed by the caregiver and a qualified healthcare provider.
A prospective, double-blinded, single arm clinical study at 14 US sites was conducted to generate safety and effectiveness data. 425 of the initial 711 subjects completed the study. 135 of the 425 (32%) subjects received a definitive Yes/No result for ASD. Study results met the effectiveness criteria for positive predictive value15 (PPV) and negative predictive value16 (NPV):
PPV = 81% (63 out of 78); 95% confidence interval = 70% - 89%
NPV = 98% (56/57), 95% confidence interval = 90.6% - 99.96%
This is a good start but there is room for improvement. The performance is better in ruling out ASD but not as good in confirming a positive diagnosis. Further, 68% of the subjects did not get a confirmed Yes/No finding.
Innovative AI/ML enabled device example #4 (Ophthalmic)
IDx-DR retinal diagnostic software for diabetic retinopathy
Diabetic retinopathy is the leading cause of blindness in patients with diabetes. Diabetes causes damage to the blood vessels all over the body, including the retina. Retinal damage may lead to other complications such as diabetic macular edema (DME), retinal detachment and glaucoma17 which can cause blindness if not treated. The risk increases over time and more than half of people with diabetes may develop diabetic retinopathy.
Detection in the early stages is important to slow down the progression of retinal damage. Generally, it requires a dilated eye exam and interpretation by a specialist.
Imagine if a routine eye-exam at a primary care clinic provider could be also be used to detect signs of early retinal damage! The patient can be promptly referred to a retina specialist for further evaluation and treatment.
The IDx-DR retinal diagnostic software is like an early warning system for a diabetic patient who has been previously diagnosed with diabetic retinopathy and does not have other conditions such as glaucoma. It uses AI to analyze retinal images to detect early signs of retinal damage and to make a referral for further screening and examination by a specialist18.
A clinical study with 900 patients across 10 primary care sites provided the following results:
Sensitivity = 87%, Specificity = 90%
Positive predictive value (PPV) = 73%, Negative predictive value (NPV) = 96%
High image quality for 96% of patients with 76% of them not needing any dilation before the eye exam
Innovative AI/ML enabled device example #5 (GI/Urology)
GI Genius gastrointestinal lesion detection software
Colorectal cancer is the third most common cancer diagnosed in the United States. The American Cancer Society estimates that nearly 153,000 new cases of colon and rectal cancer will be detected in 202319.
Screening and early detection is saving lives. There has been a consistent drop in the rate of incidence, especially among older adults due to routine screening and lifestyle changes. Still, there is a need to further improve detection capability and effectiveness. It is not uncommon for many polyps to remain undetected because of their size or hard-to-reach location20.
GI Genius(TM) is an AI-powered solution to improve detectability of polyps in real time when used with high-definition colonoscopy. A 1% increase in adenoma detection rate (ADR) decreases patient’s risk of colorectal cancer by 3%21. This is a very significant improvement in detectability!
In a randomized trial with 685 patients undergoing screening colonoscopy at 3 clinics in Italy, there was a 30% increase in ADR relative to standard high definition colonoscopy. There was no impact on factors affecting procedural efficiency and false positive rates.
Key points
In closing, these examples of innovative devices highlight the impact of AI/ML enabled medical devices on improving diagnosis, medical procedure efficiency and patient outcomes. The rapid increase in the rate of AI/ML enabled devices cleared by the FDA shows a supportive regulatory approach to encourage continued innovation. While the focus of current innovation is concentrated in radiology applications, there are plenty of examples that show a broad range of potential applications.
Premarket notification regulatory pathway is also known as the 510(k) process. Technically speaking, devices authorized through the 510(k) process are “cleared” for marking and not “approved” by the FDA.
AI/ML enabled medical devices - updated list as of Oct 5, 2022
Caption Guidance - AI-guided software for capturing diagnostic quality ultrasound images
AFib: Atrial fibrillation causes a type of irregular heartbeat (arrhythmia) which results in quivering of the atria (upper chambers of the heart) instead of a regular contraction. It affects how blood flows into the ventricles (lower chambers). Blood may pool in certain areas of the atrium, especially in the left atrium appendage (LAA) and clots may form.
LAA: Left atrial appendage is a small, ear-shaped sac in the muscle wall of the left atrium.
DEN 200030: Summary of the De Novo decision for HEARTguide
See ClinicalTrials.gov: Computational simulation plan for percutaneous left atrial appendage closure
PREDICT-LAA results summary on FEops website
CDC: Autism spectrum disorder (ASD) is a developmental disability caused by difference in the brain, that can cause a child to grow up with significant social, communication and behavioral challenges. They may also suffer from anxiety, depression or attention-deficit/hyperactivity disorder (ADHD).
DEN 200069: Cognoa ASD diagnosis aid
PPV: Positive predictive value is the probability that a positive result (YES for ASD) is truly positive compared to a clinical reference standard.
NPV: Negative predictive value is the probability that a negative result (NO for ASD) is truly negative compared to a clinical reference standard.
DEN 180001: De Novo summary for IDx-DR
Key statistics for colorectal cancer - American Cancer Society
Nearly one fourth of colorectal neoplasias (abnormal cell growth) are missed during screening colonoscopies (Source: Efficacy of real-time computer-aided dectection of colorectal neoplasia in a randomized trial, Gastroenterology, 2020 Aug: 159(2): 512-520)
GI Genius Information Sheet - Source: Medtronic