How Quality Can Help Overcome MedTech R&D Challenges
MedTech innovation is essential to drive growth, but R&D productivity is slowing down. Quality has an opportunity to lead compliant innovation in a shifting market and regulatory environment.
A recent McKinsey report1 highlights challenges - and new opportunities - for MedTech leaders over the next decade in a rapidly shifting market and regulatory landscape. According to this report, while MedTech posted a “stellar run of value creation for much of the 2010’s”, the industry now faces a tough road ahead:
Since 2019, however, investor skepticism has returned; the S&P 500 has beaten medtech every year, and valuation multiples for the highest-growth companies have been more than halved .
A recent analysis of revenue and earnings growth of top 10 US-based MedTech firms supports the need for new value creation.
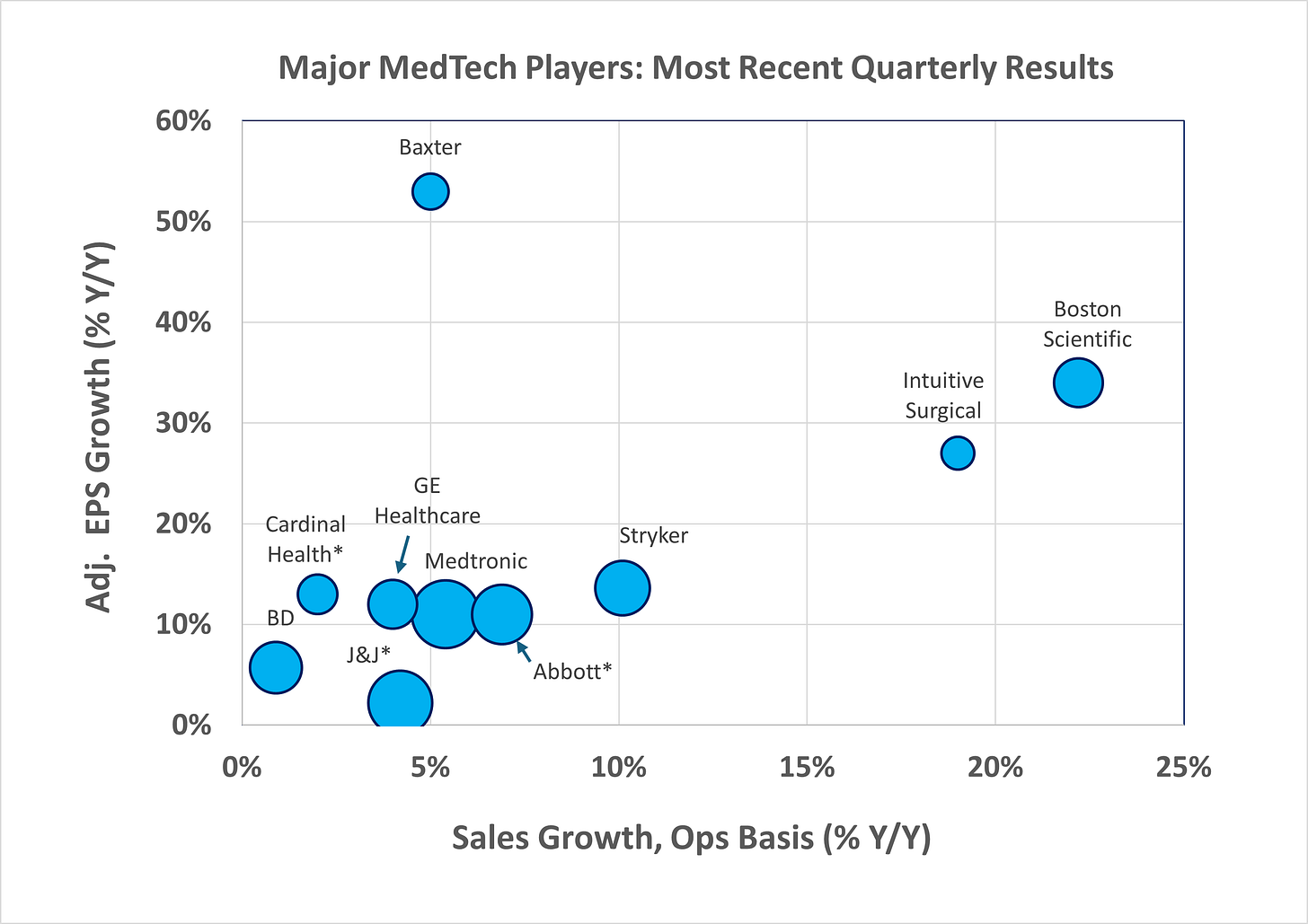
As shown in Figure 1, most of the leading MedTech companies reported operational sales growth in low-single digits, and earnings growth in high-single, or low double- digits during the most recent quarter. Stryker, Intuitive Surgical and Boston Scientific were the three breakaway companies with both sales and earnings growing double digits compared to the same quarter in prior year.
Further, MedTech R&D productivity, measured as average number of US FDA approvals per billion dollar of spend, has declined by an average of 9% per year since 2019. Incremental innovation is no longer sufficient; a transformational approach is needed to unlock sustainable growth over the next decade.
Here are a few ways Quality, Regulatory and Medical professionals can help lead this transformation.
Keep reading with a 7-day free trial
Subscribe to Let's Talk Risk! to keep reading this post and get 7 days of free access to the full post archives.



