Medical Device News Update - May, 2023
FDA approvals, warning letters and recalls issued during the month of May 2023
Dear colleagues - at the end of each month, I will share with you a summary of FDA approvals, warning letters and recalls from the prior month. My goal is to help you stay on top of these important developments in our industry without having to spend too much time searching for information across multiple sources.
Let me know in your comments below if you find this information useful.
I. Innovation
Here is a summary of De-Novo decisions, PMA approvals and 510(k) clearances in the month of May 2023. See footnotes for links to additional information.
A. De-Novos
DEN210040: VITROS anti SARS-CoV-2 total reagent pack and calibrator1
A total reagent pack and a calibrator kit is a chemiluminescent immunoassay intended for qualitative detection of total antibodies to SARS-CoV-2 in human serum and plasm. This test is intended to for use as an aid in identifying individuals with adaptive immune response to SARS-CoV-2 on or after 15 days post symptom onset.
Product Code QVP, Class II, Regulation 21 CFR 866.3983DEN210038: VITROS anti SARS-CoV-2 IgG reagent pack and calibrator2
Similar to above except for qualitative detection of IgG antibodies instead of total antibodies.
Product Code QVP, Class II, Regulation 21 CFR 866.3983DEN2200083: Stanza behavioral therapeutic for treatment of fibromyalgia3
Stanza provides digital computerized Acceptance and Commitment Therapy, a form of cognitive behavioral therapy, and is indicated for the treatment of fibromyalgia symptoms in adult patients. Fibromyalgia is characterized by widespread musculoskeletal pain, fatigue, sleep, memory loss and mood issues. Acceptance and commitment therapy (ACT) focuses on improving normal functioning by increasing the patient’s ability to act in accordance with personal values while experiencing pain and distress.
Product Code QWI, Class II, Regulation 21 CFR 882.5804DEN220027: B.R.A.H.M.S SFit-1/P1GF Kryptor test system4
This is an automated immunofluorescent assay for the quantitative determination of the placental growth factor (P1GF) and the VEGF receptor -1 in human serum and plasma. It is used as an aid in clinical assessment of risk in pregnant women hospitalized for hypertensive disorders of pregnancy for progression to preeclampsia with severe features (kidney or other organ damage).
Product Code QWH, Class II, Regulation 21 CFR 862.1602
B. Premarket Approvals (PMA) - Original only
1. P130010: VEGA steroid-eluting endocardial leads5
A drug eluting permanent right ventricular (RV) or right atrial (RA) pacemaker electrode. Drug eluting permanent RV or RA pacemaker electrodes are used with compatible pacemakers or implantable cardioverter defibrillators (ICD) or cardiac rhythm therapy (CRT) devices to deliver pacing pulses to the heart.
Product Code: NVN
2. P210036: PerClot® Polysaccharide Hemostatic System6
Perclot(R) is an absorbable hemostatic powder used in surgical procedures (except neurological and ophthalmic) to support and assist control of bleeding along suture lines or other types of bleeding when conventional procedures are ineffective or impractical.
According to the manufacturer’s website7, PerClot(R) is an absorbable polysaccharide powder which is used as supplied without any mixing step. It is applied directly to the site of active bleeding. Once hemostasis is achieved, excess powder is removed from the site.
In a clinical trial8, 145 of the 160 patients receiving up to two 5g bellows during an index procedure achieved hemostasis within 7 minutes. This result is statistically non-inferior to the control arm where 149 of 162 patients achieved hemostasis under similar conditions. Interestingly, 144 of the 160 patients achieved hemostasis within 5 minutes with PerClot(R) compared to 138 of 162 in the control group.
There was 1 all cause mortality out of 161 patients receiving PerClot(R) vs. 5 out of 163 in the control arm. Overall rate of serious adverse event was similar in the two groups.
3. P220013: TactiFlex(TM) ablation catheter and equipment9
This device is a sensor enabled, ablation catheter with a flexible tip used for tissue ablation during atrial fibrillation treatment.
Catheter ablation is used to create carefully controlled scar tissue inside the heart to restore a regular heart rhythm. Either radiofrequency (RF) wave ablation or cryoablation methods can be used. This particular ablation catheter uses RF energy.
According to the manufacturer’s website10, 99.4% acute success was achieved in a clinical trial. Acute success is defined as isolation of all pulmonary veins confirmed by entrance block by the end of the procedure.
A post-approval study11 is currently in progress.
C. Premarket Notifications (510k)
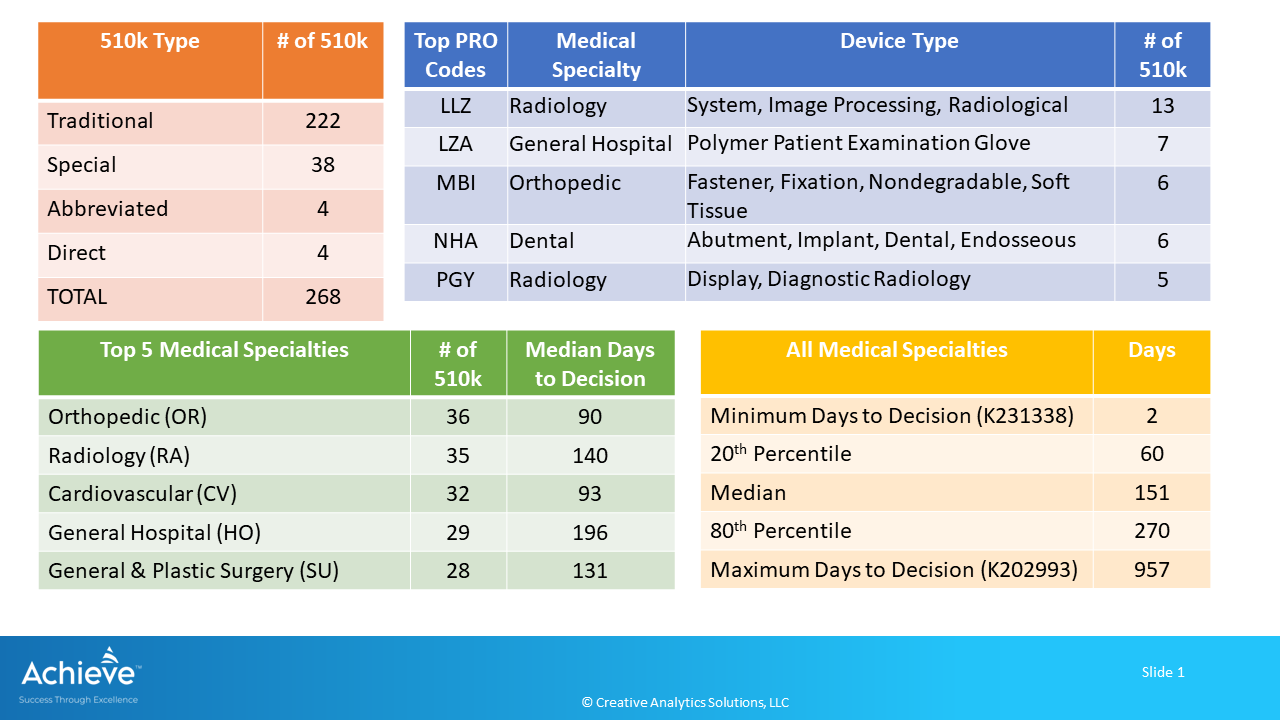
FDA cleared a total of 268 devices through the premarket notification process during May 2023. A majority of these devices (83%) were cleared through the Traditional 510k process.
Following graphic provides an overview of these 510k’s.
Here are the key highlights:
Top 5 medical specialties accounted for 60% of the total 510k (160/268) with median days to decision ranging from 90 to 196after receipt.
Orthopedic medical specialty had the highest number of 510k (36/268) with 90 median days to decision after receipt. Top 5 product codes were mainly in the radiology, general hospital, orthopedic and dental specialties.
LLZ was the top product code corresponding to radiological image processing systems.
In aggregate, days to decision ranged from a minimum of 2 days to a maximum of 957 days after receipt, with a median of 151 days. 20th percentile of the devices were cleared within 60 days, while the 80th percentile were cleared within 270days.
3M Clinpro(TM) clear 2.1% sodium fluoride treatment indicated for hypersensitive teeth was cleared in just 2 days after receipt (Product Code: LBH, 510k number K231338)
Buzzy(R) therapeutic vibrator intended to control pain associated with needle procedures and temporary pain relief from minor injuries was cleared in 957 days (Product Code: PHW, 510k number K202993)
II. FDA Warning Letters
There were 2 device related warning letters issued during the month of May 2023.
CMS 643474 iRhythm Technologies, Inc.12
Devices impacted: ZIO AT ECH monitoring system
Observations cited: unapproved use of modified device compared to original ZIO QX ECG monitoring system (K163512). Labeling violations where users were not informed of the data transmission limit. Quality system regulation violations related to CAPA, health hazard evaluations, complaints handling and MDR reporting violations.CMS 651802 Steiner Biotechnology, LLC13
Devices impacted: Bone grafting materials
Observations cited: unapproved use of modifications in Socket Graft, Socket Graft Plus, OsseoCondutct, OsseoConduct Micron, Ridge Graft Kit and Immediate Graft devices (adulterated). Quality system regulation violations related to design and development procedures, process validation, purchasing controls, acceptance activities, equipment calibration, environmental controls, and quality audits,
III. Medical Device Recalls
In the month of May 2023, FDA published 3 announcements related to
Class I recalls. This classification reflects the most serious type of recall, where the use of impacted devices may cause serious injuries or death.
ICU Medical: Replacement batteries for infusion systems14
Device use: These are large volume infusion pumps used for delivering precisely controlled dosage and rates for drugs and blood or blood products. Batteries are used when the pumps are not connected to an AC outlet.
Reasons for recall: replacement batteries were recalled due to a manufacturing defect that resulted in diminished battery life.
Patient safety impact: patients may experience serious injury or death due to interruption, under-infusion or delays in the delivery of critical fluids, blood products and medications.Draeger Medical: Seattle PAP plus and breathing circuit/anesthesia kit15
Device use: The Seattle PAP (Positive airway pressure) system provides bubble continuous positive airway pressure (CPAP) therapy to provide breathing assistance to infants. Breathing circuit and anesthesia kits are used together with ventilators during surgery or in the intensive care unit to provide breathing support for infants, children and adults.
Reasons for recall: machines and anesthesia kits were recalled after finding that glued connections may loosen before or during ventilation due to a manufacturing error. Partial or complete detachment of components and connectors may occur as a result.
Patient safety impact: Loosening or detachment of components and connectors can interrupt the breathing circuit and may cause severe injury including lack of oxygen or death.SD Biosensor, Inc.: At-home COVID-19 test kits16
Device use: at-home test for qualitative detection of COVID-19
Reasons for recall: liquid solutions in the test kit may be contaminated with bacteria.
Patient safety impact: infection from exposure to bacteria may cause illness in people with weakened immune system or those exposed to these liquids through standard handling, accidental spills or misuse of the product.
P130010 VEGA Steroid-Eluting Endocardial Leads: PMA database entry
P210036 PerClot® Polysaccharide Hemostatic System: PMA database entry
PerClot(R) information on baxter.com
NCT02359994: PerClot (R) Clinical Trial
P220013 TactiFlex(TM ablation catheter and equipment: PMA database entry
TactiFlex(TM) clinical evidence on abbott.com
TactiFlex(TM) post-approval study