Medical Device Recalls - Part 1: High Level Overview
A high level overview of 283 Class I recalls of medical devices in the last 3 years.
Medical device recalls classified by the FDA as Class I represent the most serious type. If these issues ware not addressed adequately, there is a high chance that patients and users will experience serious injuries including life threatening conditions and death. That is why it is useful to actively monitor device recalls as part of your post-market surveillance process and take timely action before it is too late.
A recent article1 presented the following findings based on analysis of 189 Class I recalls from January 1, 2018 to June 30, 2022 for moderate to high risk medical devices:
Approximately 80% of the 189 Class I recalls were for moderate risk devices (Class II) and 18% for high risk devices (Class III).
Cardiovascular medical specialty accounted for 34% of recalls, followed by Anesthesiology (21%) and General Hospital (17%). 19% of recalls involved implanted devices.
Device design was the most frequent cause for recalls (~55%) followed by issues in manufacturing/processing (~25%) and software (11%).
The median number of device units per recall notice was 4620 with around 6% of recalls involving more than 1 million device units.
125 devices (66%) were involved in multiple recalls with a median of 4 recalls issued per recalled device.
As of September 15, 2022, 50 (~27%) recalls were terminated with a median of 24 months elapsed since they were initiated.
More importantly, the authors presented the following conclusion:
Class I medical device recalls are common and affect millions of device units in use in the US. Completing the required actions for recall termination takes a significant amount of time, posing serious safety concerns to patients for a longer period.
In this article, we extend the analysis of recalls data to class I recalls reported from July 1, 2022 to May 31, 2025. Our intent is to seek deeper insights, not simply replicate the analysis and inferences reported in the above article.
Class I recalls data was extracted from Orca1.ai, a comprehensive analytics platform for life sciences.
Class I Medical Device Recalls - A high level view
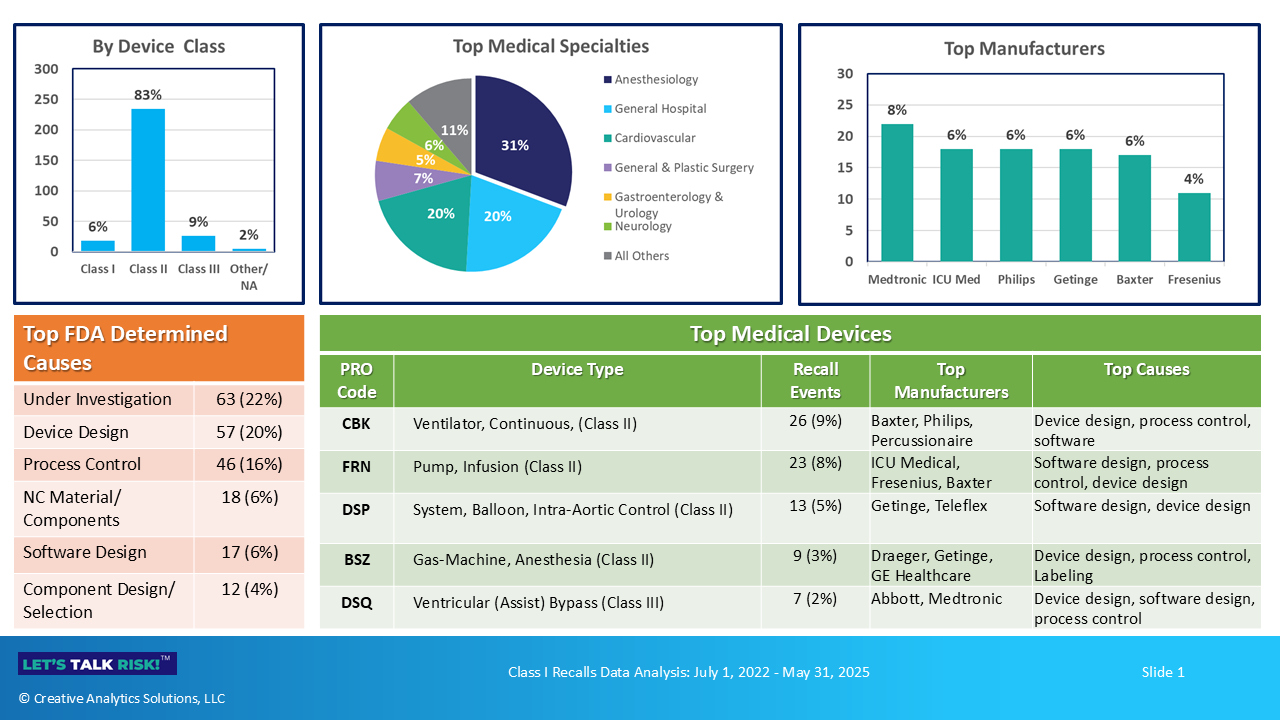
A total 283 Class I recalls were reported during the timeframe of our analysis (Jul 1, 2022 - May 31, 2025). A high level snapshot of these recalls is presented in the Figure 1.
Here are a few key highlights:
Class II (moderate risk) devices accounted for 83% (234/283) of recalls, followed by Class III (high risk) at 9% and Class I (low risk) at 6%.
Anesthesiology was the top medical specialty for recalls (87/283, 31%), followed by General Hospital (57/283, 20%) and Cardiovascular (56/283, 20%).
Medtronic had the highest number of recalls (22/283, 8%) across 16 product codes, followed by ICU Medical, Philips and Getinge, each with 18 recalls.
Device design was the top cause for recalls as determined by the FDA (57/283, 20%), followed by Process control (46/283, 16%). Nonconforming materials, software design, and component design/selection were some of the other top categories of recall issues.
Top 5 product codes representing ventilators, infusion pumps, aortic balloons, anesthesia gas machines and ventricular assist bypass, accounted for a total of 78 recall events (28%). In all, there were a total of 129 product codes associated with these 283 recall events.
Let us take a closer look
1. Top 5 Product Codes
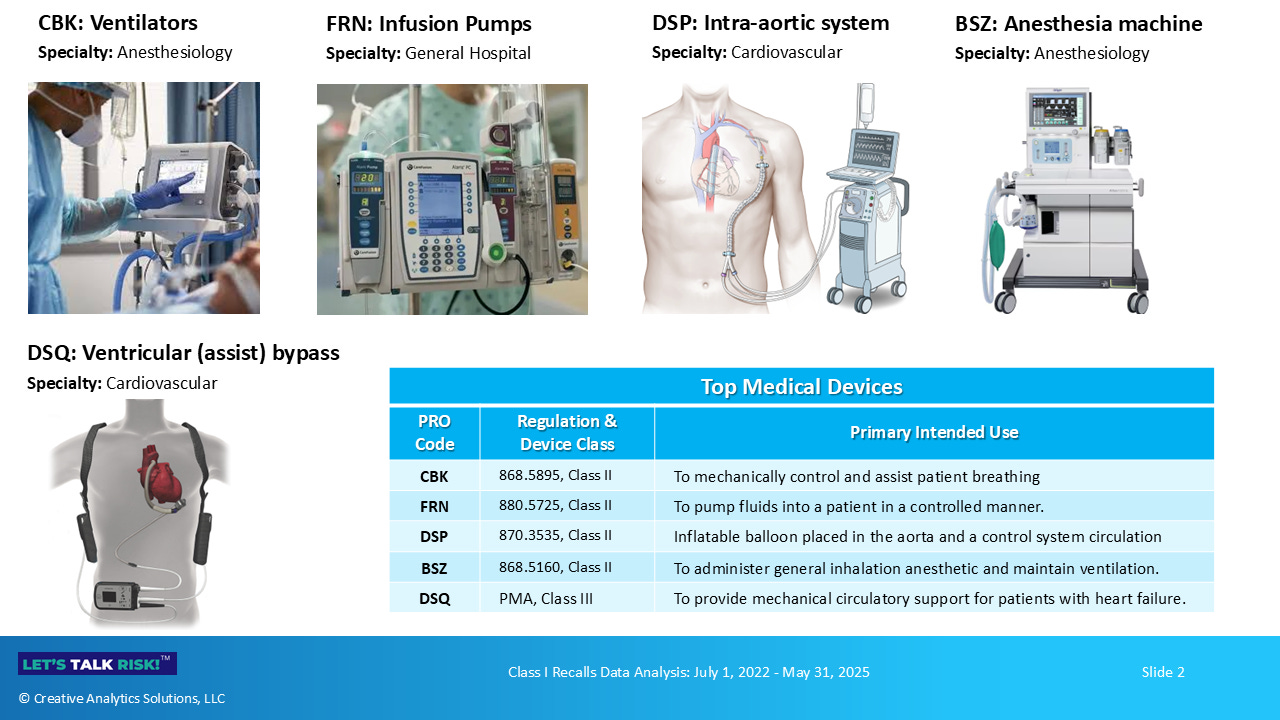
Figure 2 below profiles the top 5 device codes associated with Class I recalls during the analysis period. As noted above, devices in these categories collectively account for 28% of the recall events (78/283).
Device classification is based on its intended use and risk level2. A vast majority of medical devices are classified as either Class I or Class II, reflecting a relatively low and moderate level of risk to patients and users. These devices are subject to specific regulations (e.g. 868.5895 for ventilators under product code CBK) which outline general and special controls as well as any exemptions. Class III devices reflect the highest relative risk, and are approved through the Premarket Approval (PMA) on an individual basis. They do not have a specific regulation number associated with their product code.
Recall classification also follows a risk-based approach, with Class I recalls reflecting the highest relative degree of potential health hazard to patients3. Continued exposure to such devices pose a serious risk to patients, with a reasonable probability of a serious adverse health consequence including a life-threatening condition and death.
Device classification and recall classification can be confusing. There is no relation between the two, and as shown in Figure 1, Class I devices can also be subject to a Class I recall. Regardless of the regulatory classification of a device, it can still pose a serious safety risk to patients if it is associated with a Class I recall.
As shown in Figure 2, 4 of the top five (5) devices are classified as Class II and one (1) as Class III. Collectively, they represent a broad range of intended uses from providing breathing assistance to delivering fluids and medications and helping to improve and sustain blood circulation in patients with heart problems.
There are a total of 129 product codes representing the full range of devices involved in these Class I recalls.
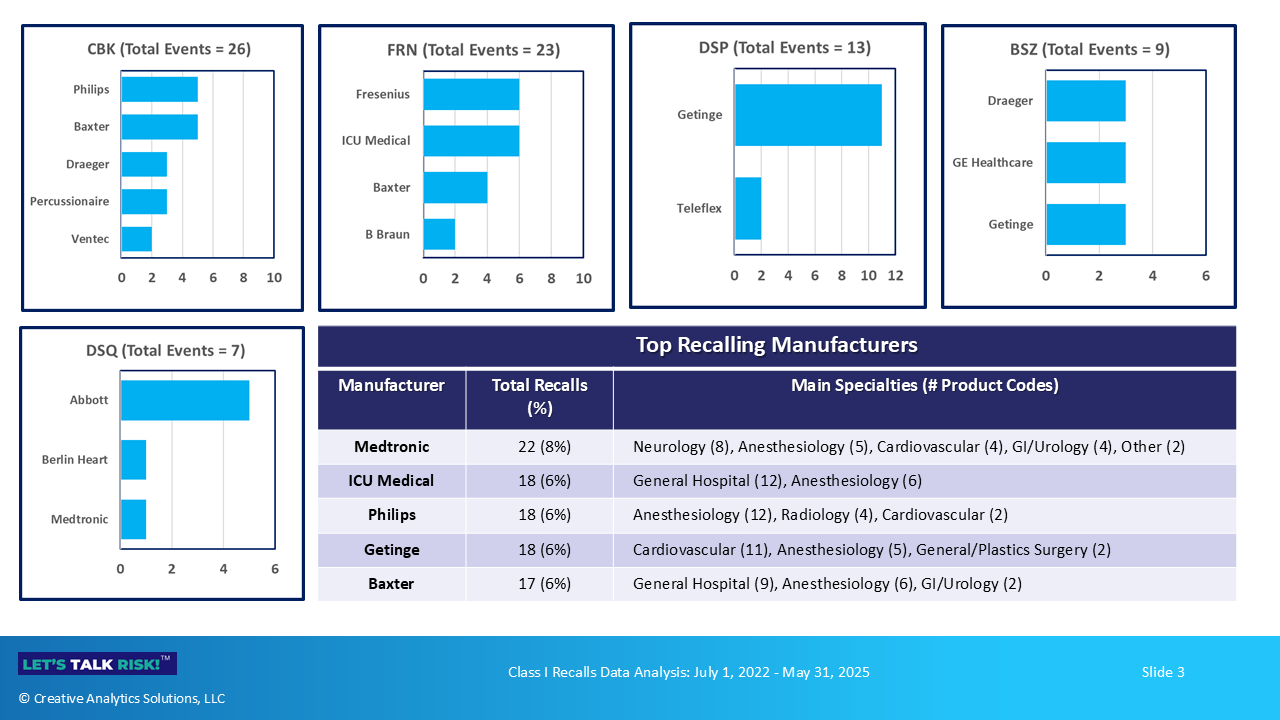
2. Top Recalling Manufacturers by Product Code
Medtronic, ICU Medical, Philips, Getinge and Baxter were the top 5 recalling firms collectively accounting for 33% of recalls. Figure 3 below shows the top recalling firms by top product codes.
Specific products involved in these recalls are listed below
Product Code: CBK (Ventilators, Class II)
Philips: Trilogy, Aeris, Garbin branded ventilators
Baxter: Life2000 ventilators
Draeger: Carina, Oxylog ventilators, NovaStar TS components
Percussionaire: Phasitron breathing circuits
Ventec: VOCSN ventilators and breathing packages
Product Code: FRN (Infusion pumps, Class II)
Fresenius: Ivenix infusion systems
ICU Medical: CADD-Solis infusion pumps and components
Baxter: Novum IQ, Spectrum, Spectrum IQ infusion pumps
B Braun: Infusomat infusion pumps
Product Code: DSP (Intra-aortic balloon system, Class II)
Getinge: Cardiosave Rescue IABP
Teleflex: Arrow Ultraflex, AutoCAT, FiberOptix, Otimus IABPs
Product Code: BSZ (Anesthesia gas machine, Class II)
Draeger: Atlan, Perseus anesthesia workstation
GE Healthcare: CARESTATUON, Avance, Aisys, Aespire systems
Getinge: Sevofluorane vaporizer, Flow-e anesthesia systems
Product Code: DSQ (Ventricular support, Class III)
Abbott: Heartmate mobile power unit, system monitor, implant kits, displays
Berlin Heart: EXCOR pediatric ventricular assist device
Medtronic: Heartware HVAD system (now discontinued)
4. Top FDA Determined Causes for Recalls by Product Code
Although a large proportion recall causes are still under investigation (63/283, 22%), the top FDA determined causes are summarized in Figure 4 below. Note that bar charts presents a product-code view for top 5 products, and the table presents the total distribution of causes.
Here are a few examples of recall issues by causes
Device Design (57 events, 20%)
CBK (Ventilators): Potential for loss of ventilation due to false alarms, loss of ventilation when transitioning from battery to AC power
FRN (Infusion pumps): Multiple failures due to battery problems, upstream occlusions, system errors, drug product leakage, incorrect flow rates, component damage
DSP (Intra-Aortic balloon system): Battery failures, unexpected shutdowns, compromised balloon catheters
BSZ (Anesthesia gas machines): Ineffective ventilation in certain operating modes, sevoflurane degradation, design related use-errors with connectors
DSQ (Ventricular support): System monitor display issues, potential leak paths, battery issues
Process Control (46 events, 16%)
CBK (Ventilators): Stuck venturi component, battery charger issues, component detachment, wrong assembly
FRN (Infusion pumps): Battery manufacturing defects, fluid ingress causing electrical failure, pneumatic valve failure modes
BSZ (Anesthesia gas machines): Component failures, pressure transducer connections reversal
DSQ (Ventricular support): Electrical component issues
Software Design and Changes (26 events, 9%)
CBK (Ventilators): Low pressure alarm issues, ambient mode after 19 days of cumulative use without warning, screen lock, air ingress detection
FRN (Infusion pumps): Various software anomalies affecting alarms, pump function, dose accuracy
DSP (Intra-Aortic balloon system): Various software anomalies affecting functionality, data transmission, loss of communication
BSZ (Anesthesia gas machines): Pressure build-up and gas delivery issues
DSQ (Ventricular support): Unexpected pump start or pump stop
Nonconforming Materials/Components (18 events, 6%)
CBK (Ventilators): Degrading capacitors, polyurethane foam emissions
FRN (Infusion pumps): Polyurethane foam emissions
DSP (Intra-Aortic balloon system): Intra-aortic balloon issues, damaged or broken central lumen, helium loss
5. Mapping multiple recalls, actions, and key issues
As shown in Figure 5 below, manufacturers have taken multiple recall actions for the same device during the timeframe of our analysis. These actions include issuing urgent safety communications (S), correction notices (C) or instructing users to discontinue using and/or return the affected devices (R).
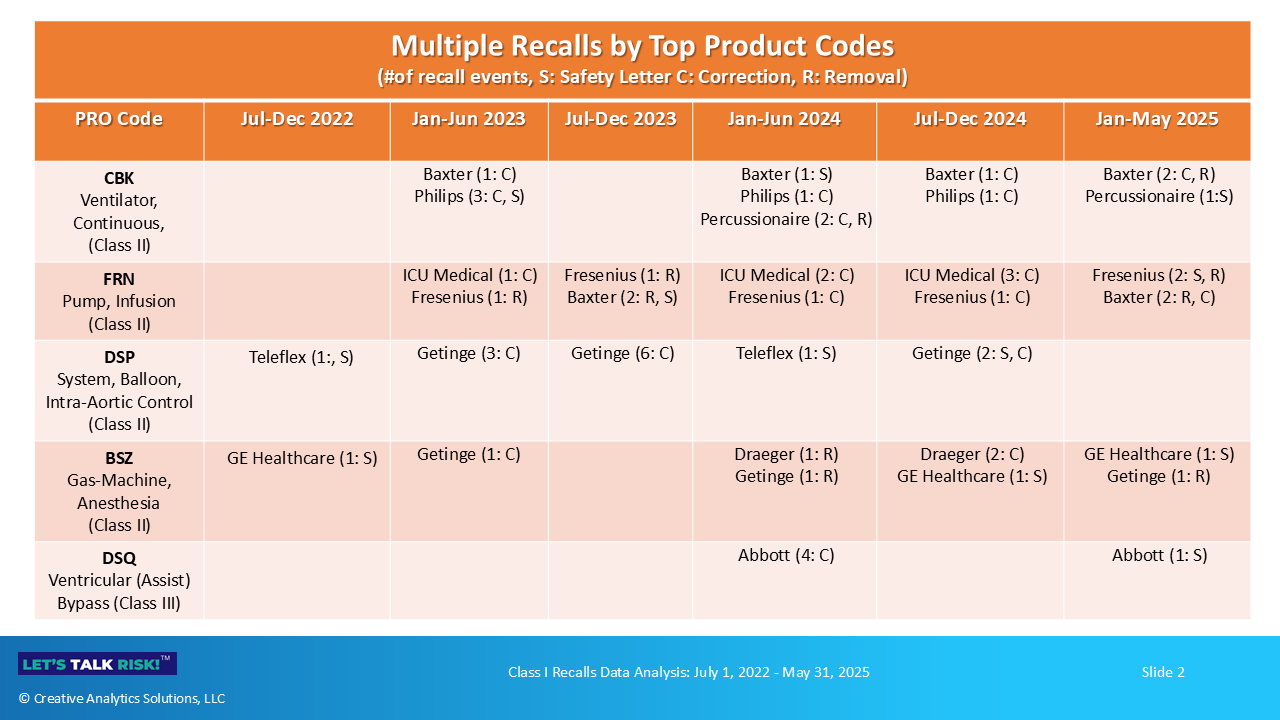
Multiple recalls of the same device may be due to different issues, or related/recurring issues that have not been effectively resolved. Here is a high-level summary of types of issues related to these recalls.
Product Code: CBK (Ventilators, Class II)
Baxter (5 recall events): battery issues, cybersecurity vulnerability, failed alarms, oxygen flow issues
Philips (5 recall events): flow measurement issues, battery depletion, debris in flow sensors, sound abatement silicone foam issues
Percussionaire (3 recall events): pressure and flow issues
Product Code: FRN ( Infusion pumps, Class II)
ICU Medical (6 recall events): counterfeit batteries, battery issues, multiple software issues
Fresenius (6 recall events): multiple software issues, valve failures, electrical issues
Baxter (4 recall events): false occlusion alarms, incorrect alerts display, missing mounting screws
Product Code: DSP (Intra-aortic balloon system, Class II)
Getinge (11 recall events): software corrections, unexpected user-reported alarms, unexpected pump shutdown, battery issues
Teleflex (2 recall events): battery issues, balloon inflation issues
Product Code: BSZ (Anesthesia gas machine, Class II)
Draeger (3 recall events): unexpected shutdown, piston ventilator issues
GE Healthcare (3 recall events): ventilation issues, incorrect connections
Getinge (3 recall events): software issues causing low pressure, sevoflurane degradation
Product Code: DSQ (Ventricular support, Class III)
Abbott (5 recall events): mobile power unit (MPU) issues, screen display issues including unresponsive buttons, seal interface issues causing leakage, software issues, outflow graft issues
In Closing - Key points
A high level analysis of Class I recalls from Jul 1, 2022 to May 31, 2025 highlights the following key points:
283 total Class I recalls in the analysis period (~ 3 years), with majority of them related to Class II (moderate risk) medical devices.
Anesthesiology medical specialty representing continuous use ventilators had the highest number of Class I recalls, followed by General Hospital and Cardiovascular.
Top 5 product codes were CBK (Ventilators) FRN (Infusion pumps), DSP (Intra-aortic balloon pump systems), BSZ (Anesthesia gas machines) and DSQ (Ventricular assist bypass).
Top 5 companies were Medtronic, ICU Medical, Philips, Getinge and Baxter.
Top 5 causes as determined by FDA were Device Design, Process Control, Software Design and Changes, Nonconforming Materials/Components and Component Design and Changes
Multiple recalls of the same device by a manufacturer highlight broad ranging issues, some of which closely related or recurring, suggesting unresolved chronic issues with these devices.
Future articles in this series will dig deeper into these recalls and underlying issues. Stay tuned!
Characterization of US Food and Drug Administration Class I Recalls from 2018 to 2022 for Moderate- and High-Risk Medical Devices: A Cross-Sectional Study, Mooghali et. al., Medical Devices: Evidence and Research 2023:16 111–122, Published 19 May 2023.
FDA: Classify your medical device, website accessed Jun 26, 2025.
FDA: Recalls, corrections and removals (Devices), website accessed Jun 26, 2025.





Naveen – this is another masterful analysis and a deeply useful article. It’s be interesting to consider what hypotheses this data supports regarding product changes and postmark experience. For example, does the data predict that certain device types are more susceptible to recall because those products iterate more often?