Dear colleagues - I am super excited to launch a new series called Tools and Tips as part of the Let’s Talk Risk! newsletter.
The idea is to highlight publicly available resources to help you learn more about medical devices. The good news is that a lot of information is available on FDA’s website. The bad news is that it is scattered in different databases and not easy to find!
In these articles, I will highlight a specific resource and show you how you can use it to gather information about medical devices for your risk management activities.
Let me know in your comments below if you find this information useful. Feel free to also suggest other tools and tips of interest to you.
A vast majority of medical devices are cleared by the FDA for marketing in the United States through the 510(k) premarket notification process1. This article highlights a searchable 510(k) database available on FDA’s website2. It is not intended to be a comprehensive guide, but it will help you get started with a couple of illustrative examples.
The 510(k) premarket notification database
When you access the 510(k) premarket notification database (see link in note 2 below), you will see the following screen:
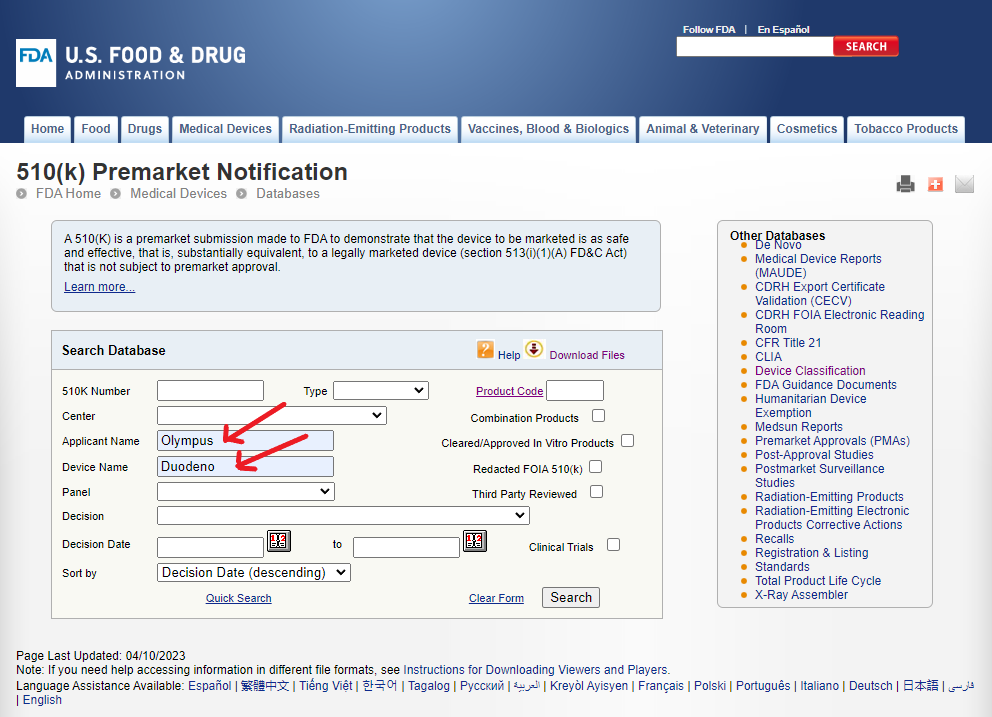
You can search this database using a variety of information applicable to each field. In the example below, we will start as if we don’t know anything except the name of the device and its manufacturer.
Example 1: searching the 510(k) database with device name and manufacturer
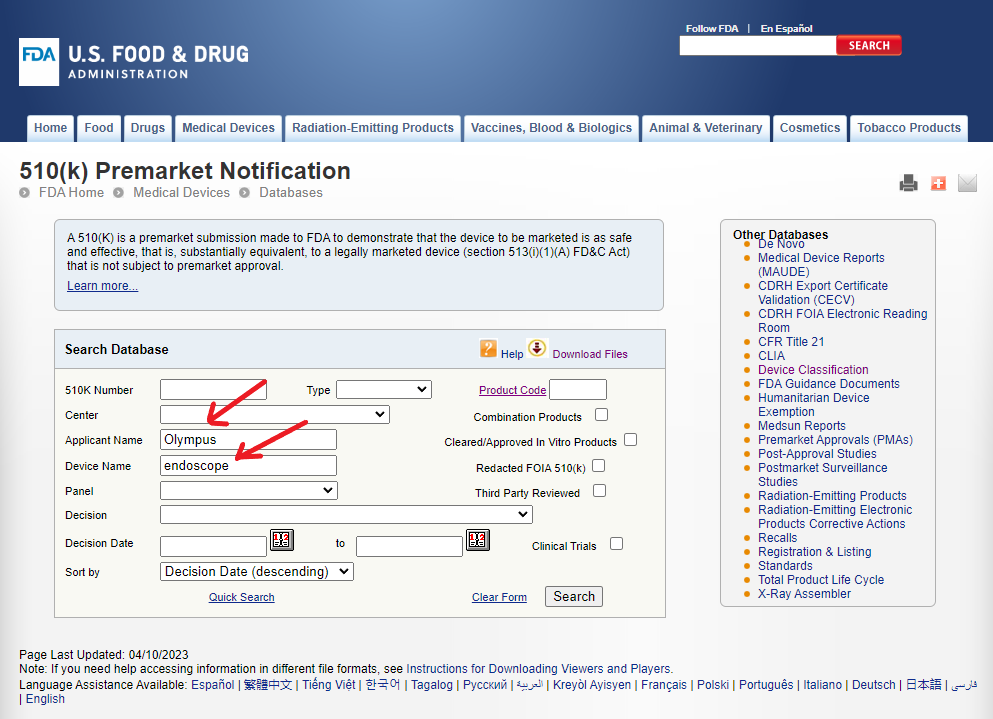
Recently, the FDA issued a warning letter to Olympus, a manufacturer of endoscopes and endoscope accessories3. Let us say we are interested in learning more about the FDA cleared devices manufactured by Olympus in this category to get some context about the warning letter. As shown in the figure below, we can enter this information in the corresponding fields and search.
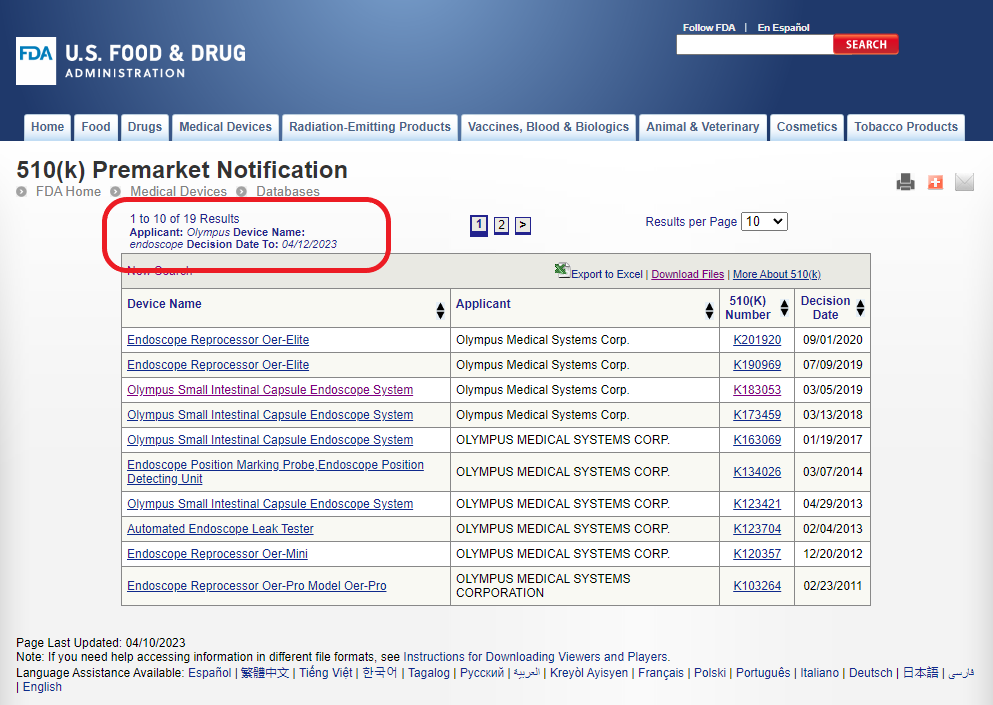
In the case the search results show a total of 19 different 510(k)s for these devices:
Hyperlinks in each Device Name or 510(k) number in the figure above provide more details.
Example 2: searching the 510(k) database for a specific device type and manufacturer
In the same warning letter, FDA cites a more specific device/accessory type as Single-Use Distal Covers for Duodenoscopes and Single-Use Suction Valve Accessories for Bronchoscope.
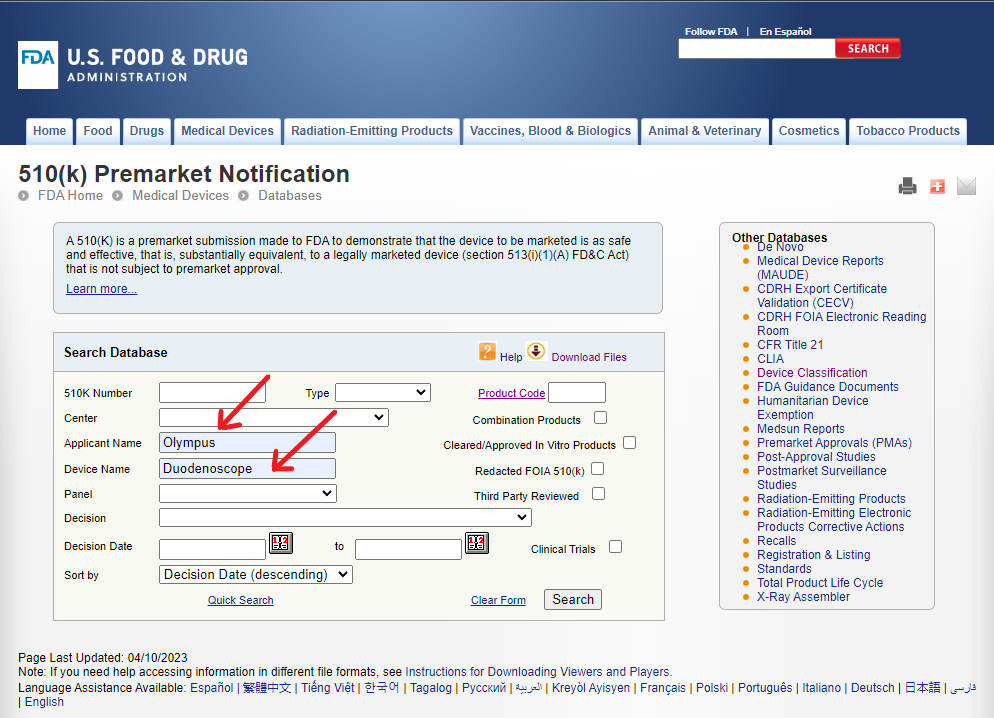
Let us now search for the term “Duodenoscope” under Device Name as a more specific device type.
This search does not show any results!
We know that the database contains many different 510(k)s assigned to Olympus, however, there are none that contain the term “Duodenoscope” in the Device Name.
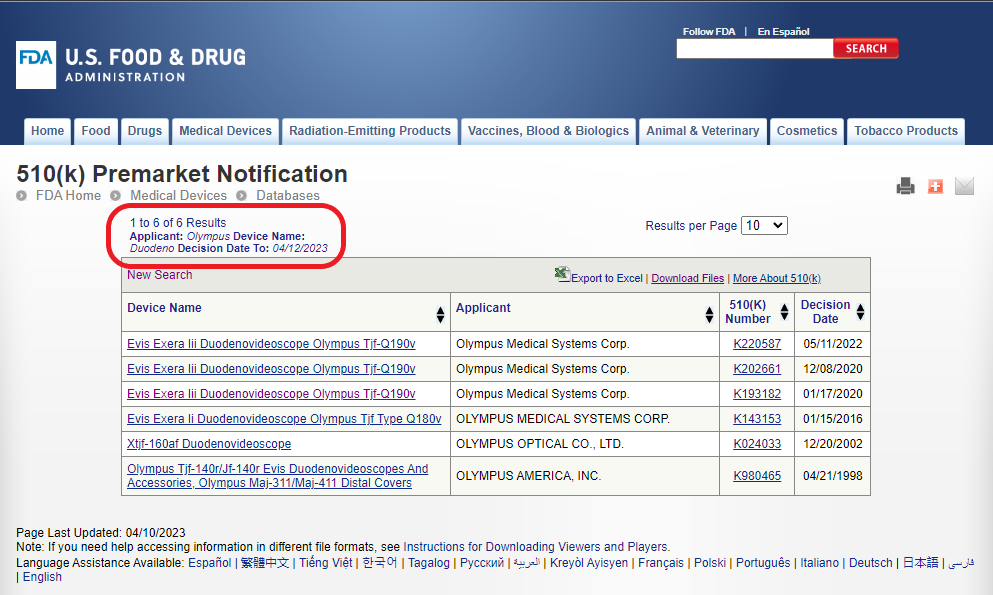
Maybe these devices are officially known by another name? What happens if we truncate the term to “Duodeno”?
Now, the search results in 6 different 510(k)s for devices that have “Duodeno” in their name. Notice that the official name is “Duodenovideoscope”. This is why we did not get any results when we searched for the term “Duondenoscope”.
Again, we can get more details on each 510(k) from the hyperlinks in the Device Name or the 510(k) number.
Example 3: searching the 510(k) database with a specific model number and manufacturer
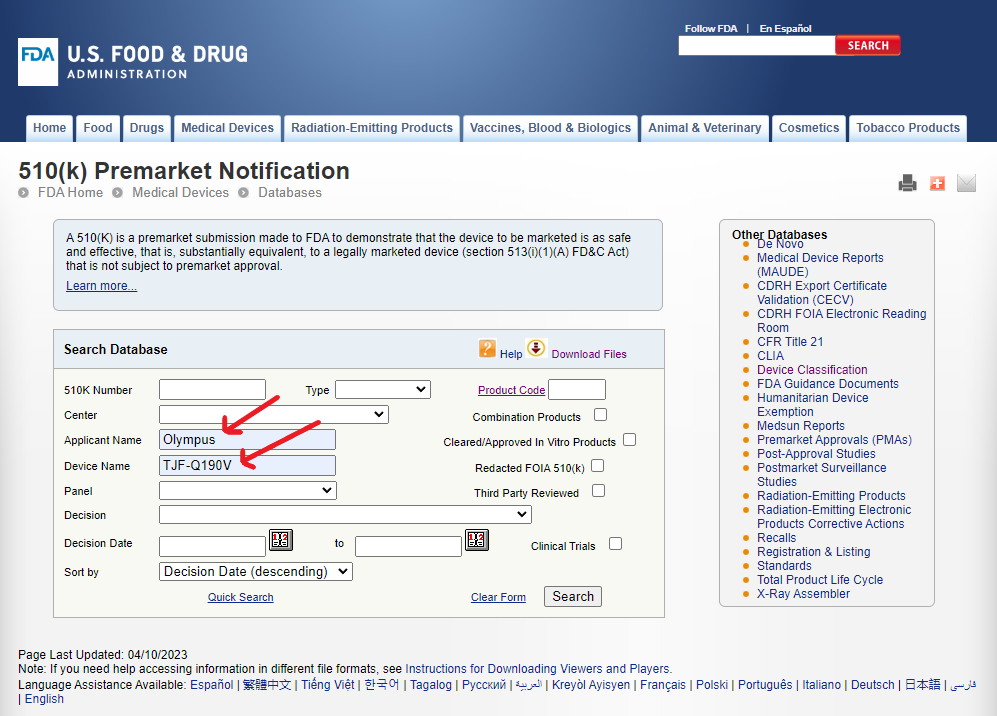
In the same warning letter, the FDA cites a specific model number for the accessory under investigation. It is called the “distal end cover” model number MAJ-2315. However, we are not going to find a 510(k) if we search using the accessory model number in the Device Name. We will need to make some educated guesses about which model of the Duodenoscope may have been filed with this specific accessory.
As shown in figure 6, TJF-Q190V seems to be the most recent model in the Evis Exera III series of duodenoscopes from Olympus. The MAJ-2315 accessory is the latest innovation because it is supposed to be a single use distal cover which allows better cleaning and reprocessing to minimize the risk of infection. Let us try our search for the TJF-Q190V model duodenoscope.
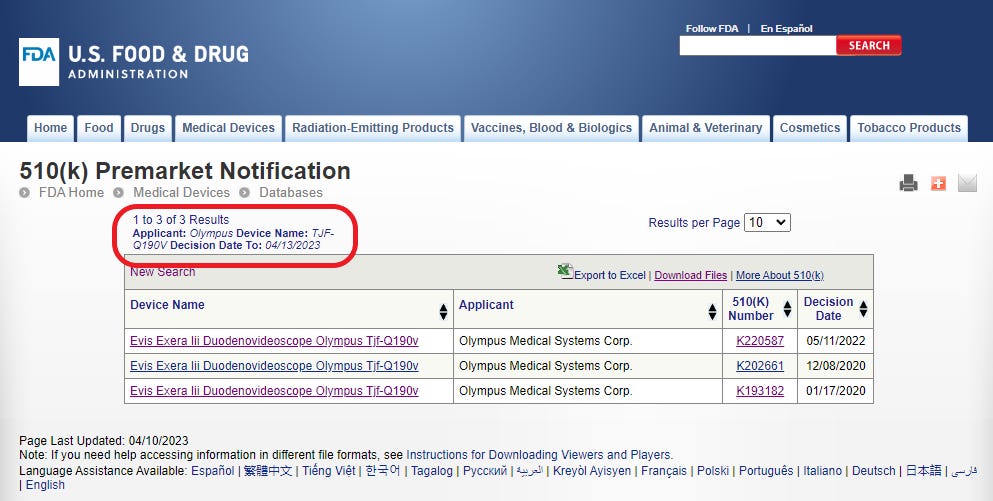
Now the search results show three different 510(k) records for the Evis Exera TJF-Q190V duodenoscopes. The earliest 510(k) was issued in January 2020 and the most recent in May 2022.
Example 4: Extracting 510(k) details from search results
We can access the detailed information for each 510(k) record by clicking on the hyperlinks in the Device Name or the 510(k) Number fields.
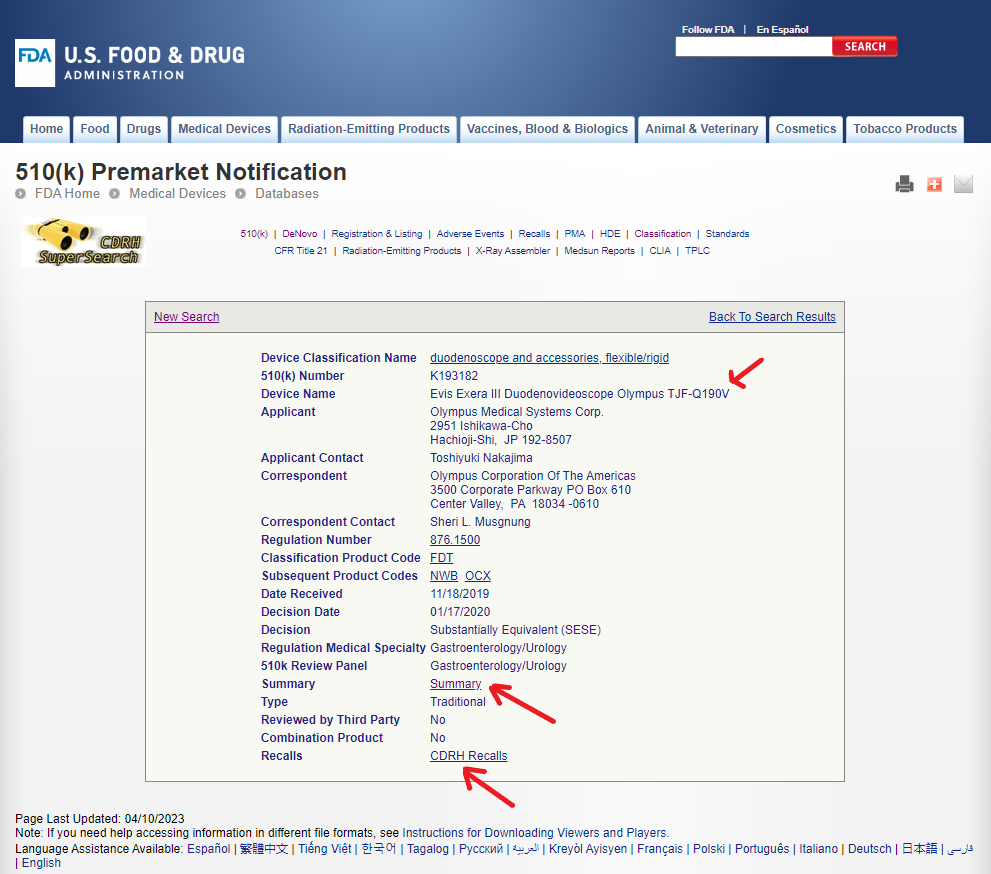
Let us click on the hyperlink for the oldest 510(k) record from January 2020.
The detailed 510(k) screen shown above in figure 9 provides more information about a specific record. In this case, we can see that 510(k) number K193182 corresponds to the Evis Exera III Duodenovideoscope TJF-Q190V manufactured by Olympus. It is classified as Product Code FDT and subject to FDA regulation number 876.1500. There are other relevant Products Codes NWB and OCX corresponding to accessories and irrigation/suction system. Note that these items are hyperlinked to more detailed information.
Two other important items in this panel are Summary and CDRH Recalls.
A downloadable PDF file is available through the hyperlink for a Summary of FDA’s decision and other device-related details, including testing to support the substantial equivalence claim. In the Summary PDF file, we can find a reference to the accessory MAJ-2315 and its intended use.
The hyperlink CDRH Recalls leads us to a page which shows a history of recalls associated with this specific 510(k).
As shown in figure 11 above, there has been one Class 2 recall linked the 510(k) record K193182. Additional details for the recall are available through the hyperlink under Product Description.
How you can use this information
As a risk practitioner, we are interested in learning about safety and effectiveness of a medical device. Publicly available information from a regulatory agency such as the FDA is the most reliable source for this purpose.
Information available from the searchable 510(k) database can be useful as a starting point to understand the regulatory history of a specific medical device. Here are a few examples of how this information can be used:
Finding a predicate device for a new medical device under development based on similar intended use.
FDA regulations and product classification codes that may be relevant for a new medical device.
Comparison of technological characteristics and type of testing done to support the claim of substantial equivalence.
History of changes in a specific medical device.
History of regulatory actions such as recalls.
Did you find this Tools and Tips article useful? Please share your opinion in this poll.
The 510(k) premarket notification leads to “clearance” of a medical device for marketing by demonstrating substantial equivalence to a legally marketed device that is not subject to premarket approval (PMA). Therefore, any device “cleared” through the 501(k) process is not “FDA approved”. It is simply permitted to be marketed on the basis of substantial equivalence.