A fast-growing frontier: AR/VR driving innovation in MedTech
Applications of Augmented and Virtual reality are growing rapidly in MedTech. Here is an overview of recent FDA approvals and key insights.
Recently, the US FDA published a list of 69 AR/VR based medical devices that are now authorized for marketing in the United States1. This article presents a brief summary of innovations in this rapidly evolving field.
The difference in Augmented Reality (AR) and Virtual Reality (VR) is subtle, but important. It is useful to note that a vast majority of authorized devices are currently based on AR.
Augmented Reality, according to the FDA, is "a real-world augmented experience with overlaying or mixing simulated digital imagery with the real world as seen through a camera or display, such as a smartphone or head-mounted or heads-up display (HUD)".
Virtual Reality, on the other hand, provides a more "immersive experience" using a headset that completely replaces a user's surrounding view with a virtual, interactive environment.
FDA cleared the first AR/VR device in 2015, but the pace has picked up since 2019
As shown in the figure below, there has been a rapid growth of AR/VR based medical devices in the last 5 years. AR/VR applications in medical devices have grown at an impressive CAGR of 68% from 2015-2023. At the current rate, the cumulative number of AR/VR based devices is expected to reach nearly 100 by the end of 2024.
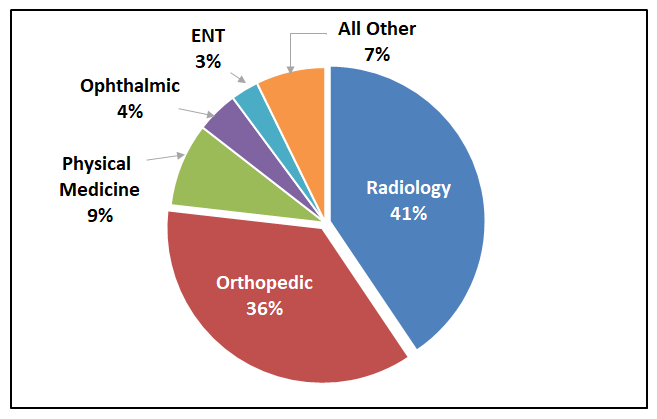
Radiology and Orthopedic specialties dominate the category
Neary 80% of the AR/VR medical device applications are in the Radiology and Orthopedic specialties. Other top areas of these applications are in Physical Medicine (9%), Ophthalmic (4%), and Ear, Nose and Throat (3%).
Top 2 product codes account for nearly 75% of AR/VR applications
A closer look at the type of AR/VR applications in medical devices reveals the reason behind the heavy concentration in Radiology and Orthopedic specialties. Only 2 product codes, as shown below, account for nearly 75% of the AR/VR applications.
It is interesting to note that product code LLZ is also a top device type for artificial intelligence and machine learning (AI/ML) based applications2. This product code is covered under FDA regulation 21 CRR 892.20503, which identifies these devices as a medical image management and processing system, intended for use by trained practitioners to detect or diagnose disease, or for patient management.
Product code OLO is covered under FDA regulation 21 CFR 882.45604, which identifies these devices to be used for precisely positioning probes or other devices within a patient’s brain, spinal cord, or other parts of the nervous system.
AR/VR applications in these categories are helping to achieve better outcomes for patients undergoing complex surgeries.
Examples of AR/VR based devices
As an example, the ImmersiveView(TM), now in version 5, from Immersive Touch, Inc. (K210726, PRO Code LLZ) is a planning software that allows the user to visualize and interactively plan surgeries in virtual reality5. It is intended for use as an image segmentation system for the transfer of imaging information, which can also be used to fabricate a physical model using traditional or additive manufacturing. An additional intended use is for precise anatomical measurements and treatment planning.
The NextAR(TM) Spine platform from Medacta International, SA (K233172, PRO Code OLO, JWH, PBF) is an augmented reality application intended to help the surgeon locate anatomical structures in open or percutaneous spine procedures6. It can also be used for measuring and selecting the fixation rod for the thoracic and lumbosacral spine.
Top 5 companies lead the AR/VR category in MedTech with nearly 30% of approvals
There are many companies actively pursuing AR/VR applications in medical devices, but the top 5 lead the category as shown in the figure below:
Medacta International is a privately held Swiss company with over 500 million in annual revenue, operating in over 60 countries.
Pixee Medical is a privately held French company. Founded in 2017, they are currently focused on developing high-performance computer-assisted surgical solutions, with a long-term goal of expanding into more effective treatments7.
Ceevra, Inc., is an early stage startup out of California, developing 3D modeling solutions using CT and MRI images for a broad range of surgical procedures8.
Augmedics is a Chicago based early stage startup developing augmented reality based guidance system for spine procedures. Their augmented reality based xvision spine system (R) has been used to treat over 7,000 patients and implant more than 40,000 pedicle screws across 24 states9.
Brainlab AG is a German company, focusing on digital medical technology with over 15,800 systems installed in 127 countries. It is a conglomerate of 6 other companies involved in a broad range of medical applications10.
In conclusion
Applications of augmented reality (AR) and virtual reality (VR) for medical use continue to grow rapidly. FDA has cleared 69 AR/VR based medical devices as of September 2024. Most of these applications are in the Radiology and Orthopedic space, dominated by the software for image processing and sterotaxic spinal procedures. These cutting edge technologies enable more precise location and measurement of anatomical structures and pre-operative planning for better patient outcomes.
FDA: Augmented Reality and Virtual Reality in Medical Devices, Accessed Sep 26, 2024.
FDA: 21 CFR 892.2050 - Medical image management and processing system.
FDA: 21 CFR 882.4560 - Stereotaxic instrument.
ImmersiveTouch: ImmersiveView(TM) interactive surgical planning tool. Accessed Sep 26, 2024.
Medacta: NextAR(TM Spine AR surgical application for total spine replacement. Accessed Sep 26, 2024.
Pixee Medical Company, About Us webpage, accessed Sep 26, 2024.
Ceevra, Inc. Website, accessed Sep 26, 2024.
Augmedics: xvision spine system webpage, accessed Sep 26, 2024.
Brainlab AG company website, accessed Sep 26, 2024.