ISO 14971 fundamentals: risk management file
Traceability and completeness of risk management need to be clearly demonstrated
ISO 14971, the international standard for application of risk management to medical device requires manufacturers to establish and maintain a risk management file. A risk management file (RMF) is an essential part of the risk management system1, but it proves to be quite challenging in practice, especially in satisfying the requirements of both traceability and completeness.
ISO 14971 requirements for RMF
Traceability: ISO 14971:2019 requires traceability for each identified hazard to the risk analysis, risk evaluation, implementation and verification of risk control measures and the evaluation of the residual risk (Clause 4.5).
Completeness: ISO 14971:2019 requires that risks from all identified hazardous situations have been considered and all risk control activities have been completed (Clause 7.6).
As an example, if you have identified 5 potential hazards, each associated with 5 different hazardous situations, where each hazardous situation could lead to 5 different harms, you would have at least 125 individual risk items in your risk analysis (5x5x5=125).
It is clear from this simple example that the number of hazard-harm combinations can quickly get out of control. There are many different techniques that can be used to eliminate irrelevant combinations, but it is also very important to build a documentation structure that allows us to organize outputs from different types of risk analysis in a simple, connected manner.
It is not uncommon for manufacturers to struggle in an audit when they try to demonstrate traceability and completeness using one or more Failure Mode Effect Analysis (FMEA) records.
Let us dive in to review a simple documentation structure for your RMF
It is important to appreciate that a broad range of tools and techniques such as Failure Mode and Effects Analysis (FMEA), Hazard Analysis, Fault Tree Analysis (FTA) etc. are used as part of the risk management process. As a result, information about risk identification, risk control and residual risk evaluation is spread across many different documents. This is one reason why it becomes challenging to demonstrate traceability and completeness of your risk management activities.
Try this simple documentation structure
If you are looking for a medical device risk management file (RMF) example, this simple documentation structure can help you. Note that this is not the only way to organize your RMF. But this has worked well in my practice with multiple clients.
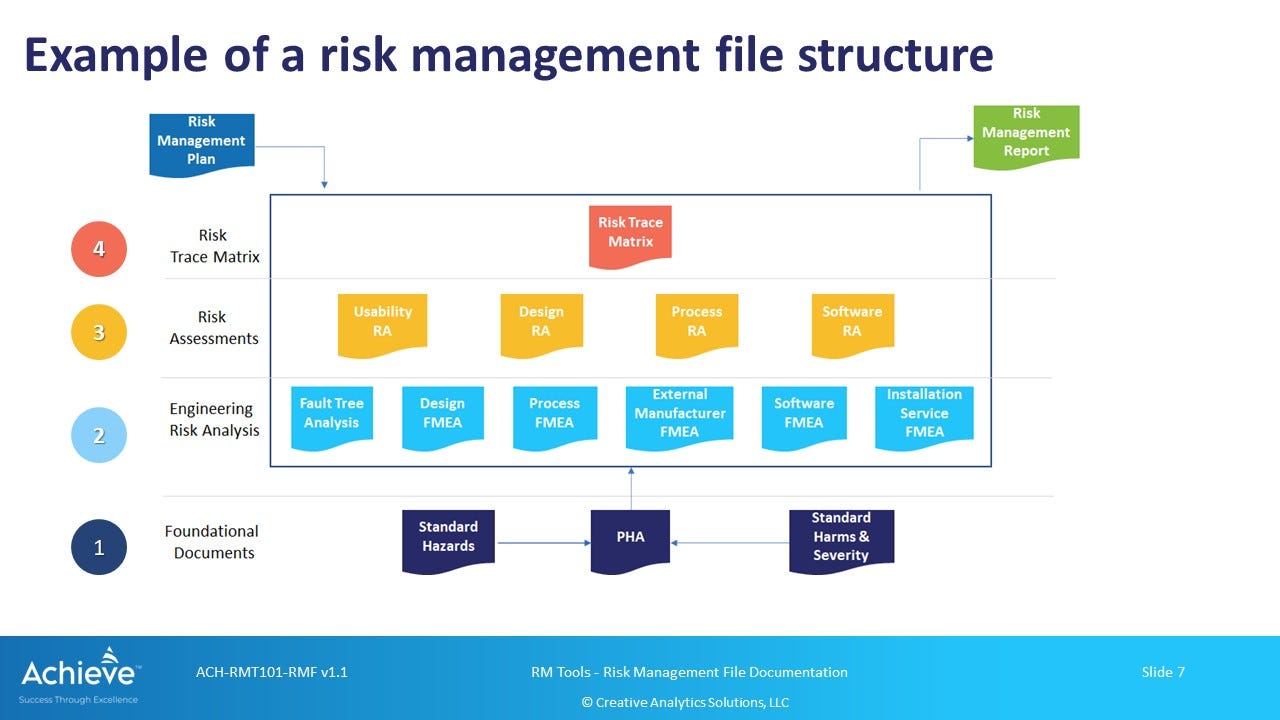
As shown in the figure above, the Risk Trace Matrix is the single record that demonstrates both traceability and completeness of the risk management process in the context of a specific risk management plan (RMP).
The other two key components of the RMF are the risk management plan (RMP) and the risk management report (RMF). Records related to specific risk management activities are shown at levels 2 and 3, which should also be included in the RMF.
If you organize your documentation structure in this way, you will find compliance to ISO 14971 requirements will be very easy! Plus you will be able to effectively communicate the status of your risk management activities with your key stakeholders throughout the device lifecycle.
Level 1: Foundational Records
A standards hazard table, a preliminary hazard analysis (PHA) and a standard harms table are the three foundational records that can be used as a starting point for a portfolio of diverse medical devices. They are not explicitly included in the RMF for a specific medical device (or family) but may be referenced in the RMP.
Level 2: Failure Analysis Records
At level 2, you may have records from different types of failure analysis. As an example, you may have dFMEA (design), pFMEA (process), sFMEA (software), uFMEA (use-misuse) etc. You may also have one or more outputs from a Fault Tree Analysis (FTA).
However, it is very important to note that these records represent analysis of potential failures, and appropriate mitigating controls. They do not represent risk analysis in the context of ISO 14971 because you are not analyzing the risk of harm in these activities.
Certainly, potential failure modes, or combinations thereof, may be related to one or more hazard-harm combinations, but not always. Therefore, wherever appropriate, you can indicate a link to hazard-harm in these records. This linkage is useful to then aggregate all risk control measures for each hazard-harm combination in the risk assessment records (Level 3).
Level 3: Risk Assessment Records
At level 3, you are now compiling information from underlying failure analysis activities in the context of specific hazard-harm combinations reflected in your hazard analysis.
Remember, risk assessment involves not only risk analysis (identification + estimation), but also risk evaluation base on criteria defined in the RMP. It also shows the residual risk level for each individual risk, and benefit-risk analysis if applicable.
At this level, you can have a Design Risk Assessment (DRA) mapped to a dFMEA, a Process Risk Assessment (PRA) mapped to a pFMEA, a Software Risk Assessment (SRA) mapped to a sFMEA, and a Use-Error Risk Assessment (URA) mapped to a uFMEA.
This separation is required because each hazard-harm may be linked to one or more potential failure modes in the underlying mapping record. Further, completeness of risk controls for a specific hazard-hazardous situation-harm combination can only be demonstrated when you aggregate all of the risk controls that are effectively implemented for related failure modes and/or use-errors.
Level 4: Risk Trace Matrix
At the highest level 4, you are now aggregating the outputs from the risk assessments at level 3. This is the single, overall record that represents the cumulative output from your risk management activities.
It may appear highly burdensome, and possibly redundant, to establish and maintain this documentation structure in a manual process that mainly utilizes Excel worksheets. However, this process can be easily automated and streamlined using commercially available relational database software.
Even in a manual operation, I have successfully deployed this documentation structure for a portfolio of multi-billion dollar medical devices supported by a small risk management team.
Lifecycle management using a simple checklist and a quality plan
Another area of practical difficulty is to maintain multiple RMFs throughout the product lifecycle.
Medical devices generally go through a lot of changes during their lifecycle in response to the experience gained during the post-market phase. It is not uncommon to have multiple cycles of design changes to fix newly discovered hazards, hazardous situations and/or device malfunctions.
Knowing exactly which record is included in your RMF, and for what purpose, is very important. Download this free checklist that provides detailed guidance on specific documents/records to satisfy the requirements of each clause in ISO 14971.
Finally, establishing a Quality Plan is very useful to manage changes to documents and records. Remember that your RMF must be current and complete at all times. Therefore, all documents and records in the RMF must be updated in a timely way.
This can prove to be very burdensome! Therefore, it is best to establish a risk-based approach to updating RMF documents through a Quality Plan. As an example, activities related to newly identified hazards, hazardous situations, harms and safety-critical device failures should be given a higher priority. Minor editorial changes and/or format changes may be done in bulk, at a lower frequency. However, it is very important for you to clearly outline your risk-based approach and document update frequency in the Quality Plan.
In Conclusion
Establishing and maintaining a risk management file (RMF) is not an easy task. It becomes especially burdensome when you have large portfolio of diverse medical products. Here are a few tips and best practices:
Build a solid understanding of ISO 14971 requirements.
Establish a simple and connected documentation structure to demonstrate both traceability and completeness.
Establish a Quality Plan with a risk-based approach to updating documents in your RMF throughout the device lifecycle.
If you want to learn more, check out the Easy Risk Management File Structure mini-course for more guidance and resources on ACHIEVE2! It includes a checklist and step-by-step directions to help you comply with ISO 14971 requirements. As a bonus, you will also get tips on audit preparation.
See clause 4.5 in ISO 14971:2019.



Hi Naveen, interesting video on RMF. I would like to ask you, suppose we use PHA (not in the sense that it is preliminary, we use it as a product hazard analysis for the lifecycle), we do include all types of hazards based on usibility, foreseeable misuse and failures. Can this be considered as a complete risk assessment? If yes, does this PHA document (matrix) be considered as the Risk Trace Matrix too ? I am confused how the RIsk Trace Matrix is different from the tools that we use for assessment(which also happent ot be a similar matrix table). Thanks in advance.